
CERTIFICATE OF ANALYSIS
|
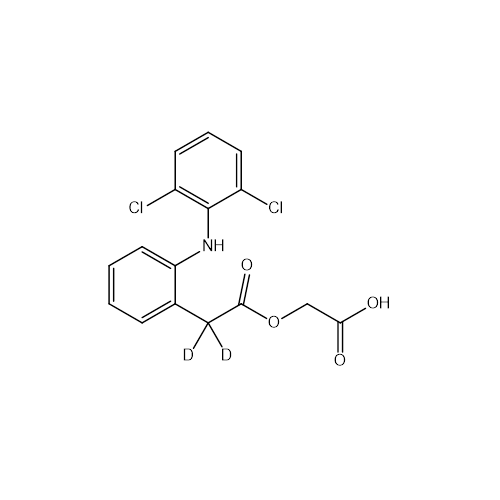
PRODUCT NAME: Aceclofenac D2
|
|
Date of Mfg: Sample COA
|
RETEST DATE: Sample COA
|
| Catalogue Number |
ACE-020
|
|
| Item/Batch Number |
Sample COA
|
| Quantity |
Sample COA
|
| CAS Number |
NA
|
| Mol. Formula |
C16H11D2Cl2NO4
|
| Molecular Wt |
356.2 g/mol
|
| IUPAC |
2-((2-(2-((2,6-Dichlorophenyl)amino)phenyl)acetyl-d2-d2)oxy)acetic acid
|
| Synonyms |
Aceclofenac D2 is supplied with detailed characterization data compliant with regulatory guideline. Aceclofenac D2 can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Aceclofenac.
|
| Long Term Storage Condition |
2-8°C with D2O vapor equilibrium
|
| Handling instruction |
Sample COA
|
| Test |
Results
|
| Appearance |
White crystalline powder
|
|
Water content by KF/TGA
|
Sample COA
|
|
% Potency = [100 - (Water content by KF)] X HPLC Purity)] / 100
|
Sample COA
|
| Identification by HNMR |
Sample COA
|
| Identification By IR |
Sample COA
|
| Mass (ESI) |
Sample COA
|
| Solubility |
|
| Purity |
NLT 98%
|
| Analysis Date (Release Date) |
Sample COA
|
| Attachments |
Sample COA
|
| Other Information |
Sample COA
|
|
Note: This material can be used as a referance standard and is for research purpose ONLY and not for human consumption
|
This is Sample COA
|