
CERTIFICATE OF ANALYSIS
|
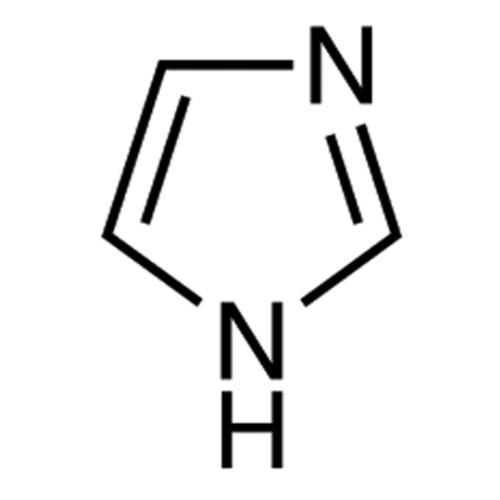
PRODUCT NAME: Clotrimazole EP Impuirty D
|
|
Date of Mfg: Sample COA
|
RETEST DATE: Sample COA
|
| Catalogue Number |
CLT-004
|
|
| Item/Batch Number |
Sample COA
|
| Quantity |
Sample COA
|
| CAS Number |
288-32-4
|
| Mol. Formula |
C3H4N2
|
| Molecular Wt |
68.08
|
| Synonyms |
Clotrimazole Imp. D (EP), Enalapril Maleate Imp. I (EP), Ondansetron Hydrochloride Dihydrate Imp. E (EP), Imidazole,Bifonazole Imp. C (EP), Enalapril Imp. I (EP), Ondansetron Imp. E (EP), Sildenafil Citrate Imp. E (EP), Sildenafil Imp. E (EP), 1H-Imidazole, Zoledronic Acid Monohydrate Imp. C (Pharmeuropa),Ph Eur Clotrimazole Impurity D, Ph Eur Ondansetron Impurity E, Ph Eur Zanamivir Impurity G,Zanamivir Related Compounds Mixture, Bifonazole Impurity C, Clotrimazole Impurity D, Enalapril Maleate Impurity I, Ondansetron Hydrochloride Dihydrate Impurity E, Sildenafil Citrate Impurity E, Zoledronic Acid Impurity C, Enalapril Impurity I, Ondansetron Impurity E, Sildenafil Impurity E
|
| Long Term Storage Condition |
2-8°C Refrigerator
|
| Handling instruction |
Sample COA
|
| Test |
Results
|
| Appearance |
White powder to crystal
|
|
Water content by KF/TGA
|
Sample COA
|
|
% Potency = [100 - (Water content by KF)] X HPLC Purity)] / 100
|
Sample COA
|
| Identification by HNMR |
Sample COA
|
| Identification By IR |
Sample COA
|
| Mass (ESI) |
Sample COA
|
| Solubility |
Soluble in Acetone, Chloroform, Alcohol
|
| Purity |
NLT 95 % by HPLC
|
| Analysis Date (Release Date) |
Sample COA
|
| Attachments |
Sample COA
|
| Other Information |
Sample COA
|
|
Note: This material can be used as a referance standard and is for research purpose ONLY and not for human consumption
|
This is Sample COA
|