
CERTIFICATE OF ANALYSIS
|
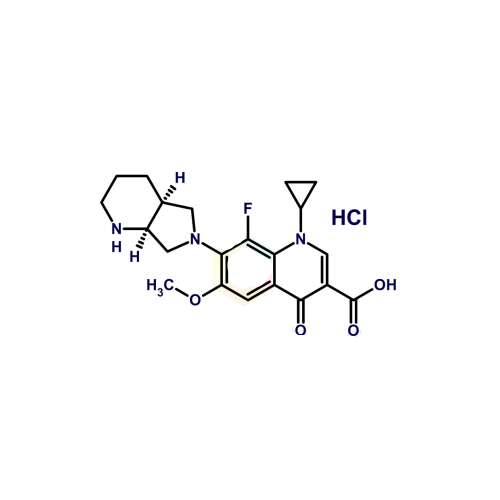
PRODUCT NAME: Moxifloxacin EP Impurity D
|
|
Date of Mfg: Sample COA
|
RETEST DATE: Sample COA
|
| Catalogue Number |
MOX-007
|
|
| Item/Batch Number |
Sample COA
|
| Quantity |
Sample COA
|
| CAS Number |
2252446-69-6
|
| Mol. Formula |
C21H25ClFN3O4
|
| Molecular Wt |
437.89
|
| IUPAC |
7-[(4aS,7aS)-1,2,3,4,4a,5,7,7a-octahydropyrrolo[3,4-b]pyridin-6-yl]-1-cyclopropyl-8-fluoro-6-methoxy-4-oxoquinoline-3-carboxylic acid;hydrochloride
|
| Synonyms |
Moxifloxacin HCl Isomer; 8-Fluoro-6-Methoxy Moxifloxacin Hydrochloride;1-Cyclopropyl-8-fluoro-6-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo [3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
|
| Long Term Storage Condition |
2-8°C Refrigerator
|
| Handling instruction |
Sample COA
|
| Test |
Results
|
| Appearance |
Yellow Solid
|
|
Water content by KF/TGA
|
Sample COA
|
|
% Potency = [100 - (Water content by KF)] X HPLC Purity)] / 100
|
Sample COA
|
| Identification by HNMR |
Sample COA
|
| Identification By IR |
Sample COA
|
| Mass (ESI) |
Sample COA
|
| Solubility |
Chloroform, Methanol
|
| Purity |
NLT 95% by HPLC
|
| Analysis Date (Release Date) |
Sample COA
|
| Attachments |
Sample COA
|
| Other Information |
Sample COA
|
|
Note: This material can be used as a referance standard and is for research purpose ONLY and not for human consumption
|
This is Sample COA
|