
CERTIFICATE OF ANALYSIS
|
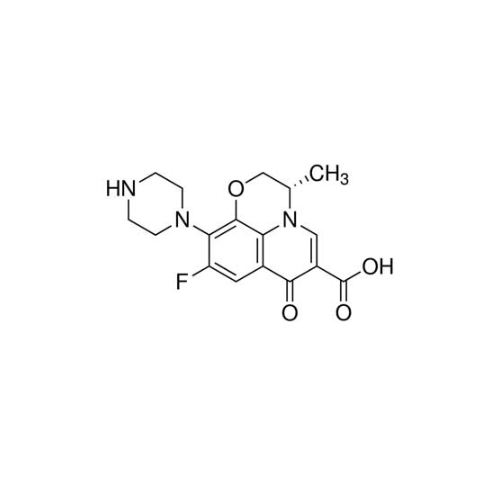
PRODUCT NAME: Levofloxacin EP Impurity B
|
|
Date of Mfg: Sample COA
|
RETEST DATE: Sample COA
|
| Catalogue Number |
LEF-007
|
|
| Item/Batch Number |
Sample COA
|
| Quantity |
Sample COA
|
| CAS Number |
117707-40-1
|
| Mol. Formula |
C17H18FN3O4
|
| Molecular Wt |
347.34
|
| IUPAC |
(2S)-7-fluoro-2-methyl-10-oxo-6-piperazin-1-yl-4-oxa-1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic acid
|
| Synonyms |
Levofloxacin Piperazine Impurity; (S)-9-Fluoro-3-methyl-7-oxo-10-(1-piperazinyl)-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic Acid; Desmethyl Levofloxacin; Desmethyl Levofloxacin
|
| Long Term Storage Condition |
store at 2-8°C
|
| Handling instruction |
Sample COA
|
| Test |
Results
|
| Appearance |
Off White Powder
|
|
Water content by KF/TGA
|
Sample COA
|
|
% Potency = [100 - (Water content by KF)] X HPLC Purity)] / 100
|
Sample COA
|
| Identification by HNMR |
Sample COA
|
| Identification By IR |
Sample COA
|
| Mass (ESI) |
Sample COA
|
| Solubility |
ethanol, DMSO, dimethyl formamide (DMF)
|
| Purity |
98%
|
| Analysis Date (Release Date) |
Sample COA
|
| Attachments |
Sample COA
|
| Other Information |
Sample COA
|
|
Note: This material can be used as a referance standard and is for research purpose ONLY and not for human consumption
|
This is Sample COA
|